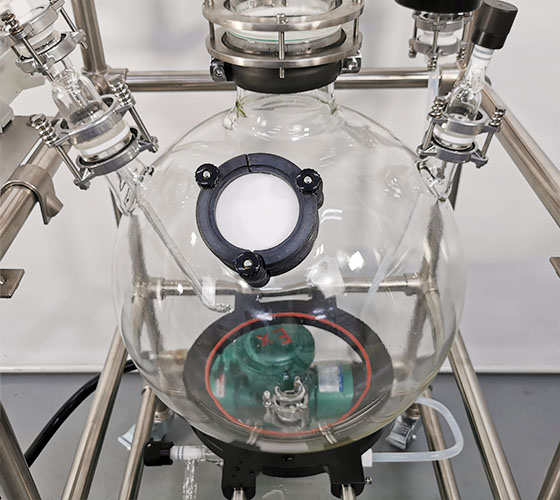



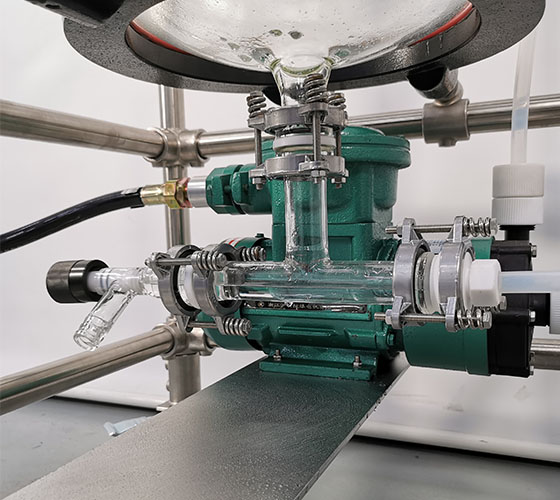











Gas scrubber
Laboratory scale equipment can be used for gas-liquid reaction or tail gas absorption in various occasions. The gas absorption device has a variety of specifications, 3l-100l liquid storage capacity. 3L compact design can be used in laboratory fume hoods, and can also be used in different fume hoods after supporting mobile racks or desktop racks. The machine consists of main absorption tank, absorption tower, glass packing, circulating pump, cooling device, spray cap, (buffer tank) and other main components.
Workflow
After starting the circulating pump, the absorption liquid is pumped into the shower from the round bottom flask through the condensation pipeline through the circulating pump, sprayed from the tower cap and re enters the absorption tank through the absorption tower. When the waste gas enters the round bottom flask and passes upward through the absorption column, it contacts with the absorption liquid with glass packing to fully mix the gas and liquid. So as to achieve the purpose of absorption. If the exhaust gas temperature is high, the condensate pipe needs to be connected to cool the absorption liquid. The device can adopt an active absorption device, such as using nitrogen as the carrier gas, or using vacuum to guide through the absorption device.
matters needing attention
The washing liquid contained in the washing cylinder shall be selected according to the nature of the purified gas and impurities. Acidic impurities, usually alkaline detergent (such as sodium hydroxide solution); Alkaline impurities, acid detergent can be used (such as Ming acid lotion); For oxidizing impurities, reducing detergent (such as pyrogallol solution) can be used. For reducing impurities, oxidizing detergent (such as potassium permanganate solution) can be used. When selecting detergent (a solution), the following two points should be ensured:
① The solute in the detergent can react chemically with the impurity gas, so that the impurity can be transformed into precipitation or dissolved as a soluble substance. Alternatively, the solvent in the detergent can fully dissolve the impurity gas, so as to achieve the purpose of removing the impurity gas.
② The main gas has low solubility in detergent and is not greatly absorbed by detergent. For example, when chlorine containing hydrogen chloride is washed with saturated salt water, hydrogen chloride is largely dissolved in salt water, and chlorine is not easily soluble in saturated salt water, so as to achieve the purpose of removing impurity gas. For example, when carbon dioxide containing hydrogen chloride is washed with concentrated sodium bicarbonate, hydrogen chloride reacts with sodium bicarbonate It is fully absorbed, carbon dioxide does not react with sodium bicarbonate, and its solubility in the solution is small, so as to achieve the purpose of removing impurity gas. If sodium carbonate solution is used, because carbon dioxide reacts with sodium carbonate solution to produce sodium bicarbonate, carbon dioxide is also fully absorbed by the solution, this method is not desirable.
Model |
QYWQ-10 |
QYWQ-20 |
QYWQ-30 |
QYWQ-50 |
Capacity of gas bottle |
10 |
20 |
30 |
50 |
Lid of port |
5 |
|||
Circulating pump |
Optional anti-corrosion fluorine plastic material |
|||
Condenser |
Thestandard configuration |
|||
Frame |
304 stainless steel |
|||
Voltage |
220V/50Hz |
|||